

Only when coupled with other acids, does alcohol become acidic? The chemical formula for ethyl alcohol, you’ll notice that it’s CH 3CH 2OH. The alcohol phenol is the lone exception to this rule, as it cannot be basic. When alcohol reacts with other strong bases, it produces OH –, which is a basic compound. When alcohol is mixed with another strong base, such as NaOH, it becomes a base, and this is the most typical result for ethanol, indicating that it is more commonly utilized as a base than an acid. Other kinds of alcohol include isopropyl alcohol, most popularly known as rubbing alcohol. When alcohol reacts with water, it does not form H + or OH –, indicating that it is neither acidic nor basic.
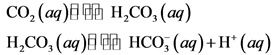
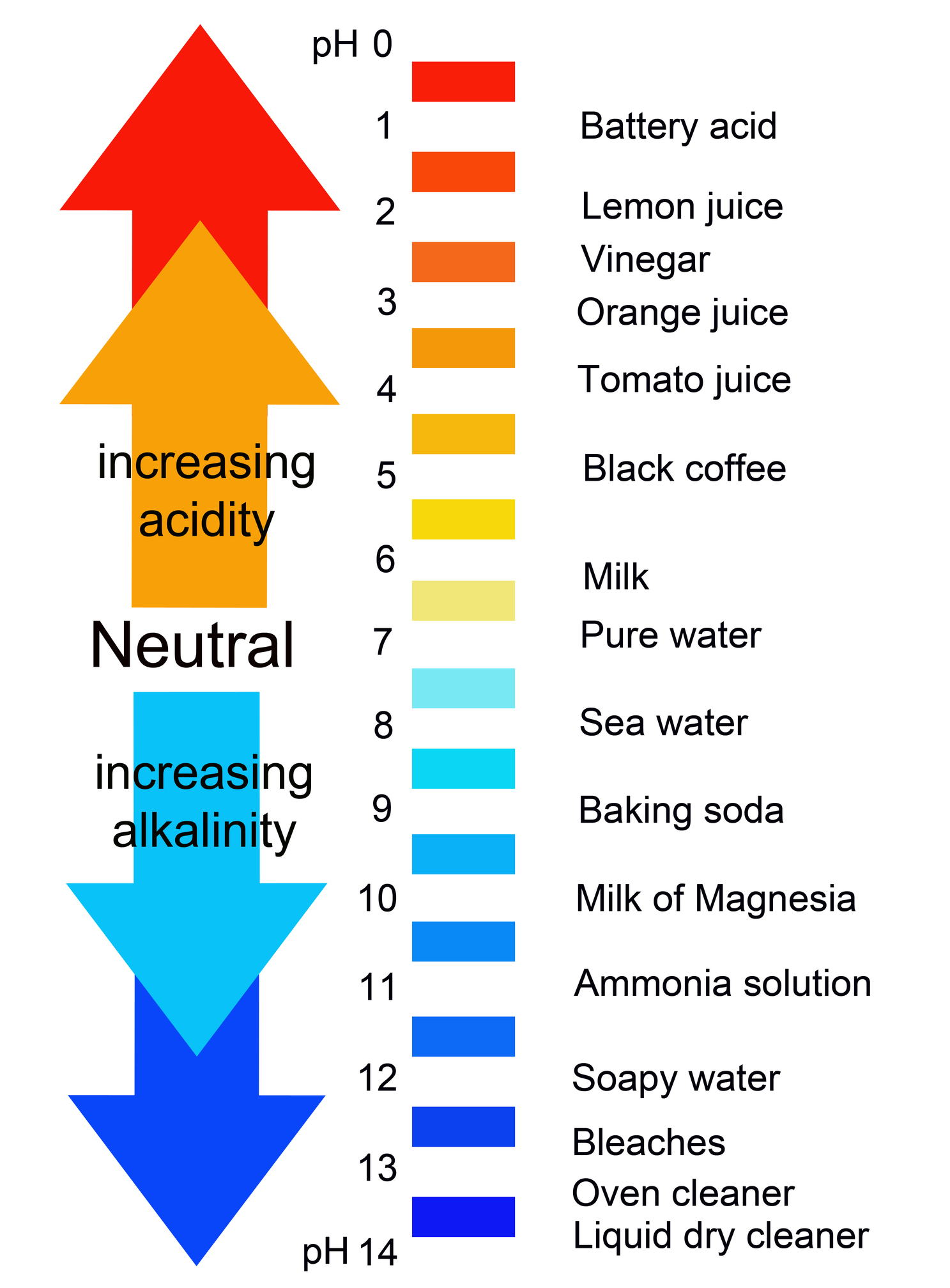
Acids are classed on a scale between 0 and 7, while bases are categorized between 7 and 14. Seawater, eggs, urine, and milk are all near-neutral substances. The only liquid that is completely neutral is pure water. Water, like alcohol, is a neutral substance. To avoid splashing, be sure the tip of the buret is in the flask (See Figure 2). Place the flask on a sheet of white paper under the buret containing the base solution. In the case of alcohol, however, an acid is defined as a chemical that releases hydrogen (H +) ions in an aqueous solution, whereas a base releases hydroxide (OH –) ions in an aqueous solution, according to the Arrhenius definition.Īlcohol is technically categorized as a solvent because it does neither and must be coupled with an acid to be an acid or a base to be a base.Ī pH scale is used by scientists to assess whether something is acidic or basic. Swirl the flask to mix all the ingredients. This hypothesis has numerous sides and levels. In the first standardization the molarity of a sodium hydroxide solution (NaOH) will be determined by titrating a sample of potassium acid phthalate (KHP HKC8H. The Arrhenius definition is the theory and science behind alcohol is neither acid nor base. Acids are classed on a scale between 0 and 7, while bases are categorised between 7 and 14. If the pH is greater than 7, the compound is. If the pH is less than 7, the compound is acidic. Or you could determine the pH of the solution using a pH meter. If you have a solution of the compound on hand, then you can use litmus paper, as someone already said. However, it can act as either, according to the aim of the reaction and the materials it is combined with.Ī pH scale is used by scientists to assess whether something is acidic or basic. There are different ways you can tell whether a compound is an acid or a base. Alcohol, which is sometimes referred to scientifically as ethanol, is neither an acid nor a base.


 0 kommentar(er)
0 kommentar(er)
